
Integrated Strategies for Management of Spotted Wing Drosophila in Organic Small Fruit Production
DOWNLOADDecember 13, 2016 - Heather Leach, MSU Extension
Introduction
Spotted wing Drosophila (SWD) is an important pest of berries, cherries, and some thin-skinned grape varieties. The berry crops at greatest risk are raspberries, blackberries, strawberries, and blueberries. For many berry and small fruit growers, SWD has rapidly become their most critical insect pest. SWD is native to Asia and was first detected in the Midwest in 2010. It is now well-distributed throughout the United States. Female SWD have a saw-like egg laying device that can cut into ripe or ripening fruit and deposit an egg (Figure 1). The resulting larvae degrade the fruit from the inside, increasing the risk of fungal pathogens and other pest damage. Larvae go through three larval stages within the fruit. The larger larvae are visible to the naked eye, creating concern of noticeable fruit contamination.

Fig. 1. Spotted wing Drosophila (SWD), male (top left), female (top right), and the females serrated ovipositor (bottom). Actual size of the fly is shown in the top left corner.
In Michigan’s southern peninsula, first SWD fly activity is typically in mid-June to early July and the population builds through the summer as temperatures continue to rise. Highest densities of SWD occur in August and September, so SWD is especially problematic for later-season berry crops, including blackberries, fall raspberries, ever-bearing strawberries, and late-season blueberries. Adults live for two to three weeks and females can lay more than 300 eggs, so they have the capacity for many generations per growing season. Typical IPM programs for pests including cherry fruit fly and blueberry maggot will not provide sufficient control of SWD. Its host range, fast generation time, and damage characteristics make it an extremely challenging pest to manage.
Conventional management programs rely on the frequent use of pyrethroid, spinosyn, organophosphate, carbamate and neonicotinoid insecticides. Of these chemical classes only the spinosyns are available to organic producers. This means that other control measures must be implemented to control SWD in organic berries.
Integrated Pest Management (IPM) programs for SWD consist of three major components: 1) the use of preventative pest management tactics, 2) monitoring and sampling for populations, and 3) the judicious use of National Organic Program (NOP) insecticides or other responsive pest management tactics. Growers should design IPM programs that emphasize management practices (e.g. exclusion, sanitation, resistant varieties), followed by the use of mechanical, biological or physical methods (e.g. hand removal, cultivation, release of predators/parasites, provision of predator/parasite habitat biological control), and resorting to NOP compliant insecticides only when necessary. In the case of SWD, insecticides are typically needed, but organic growers should integrate preventative cultural, physical and biological tactics into their SWD management programs as much as possible to help ensure effective control.
Monitoring, Identification & Thresholds
SWD can be monitored using a simple homemade trap or by buying traps (e.g. Great Lakes IPM). Traps should be placed in your field before fruit begins to ripen to identify if this pest is active. It is best to have multiple traps in each field, and at least one trap should be at the border of your crop, nearest a wooded edge where SWD activity may be earlier. Traps should be hung within the plant canopy in a shaded area and checked weekly. If creating homemade traps, we suggest using a 32 oz. plastic cup with ten 3/16” to 3/8” holes melted or cut within 2” of the top (an in-depth guide to trap construction can be found at http://goo.gl/yL2uo8). The small holes allow SWD to enter but prevent entry of larger insects. A yellow sticky trap can be placed on the inside for capture and faster identification of SWD.
Traps must be baited to attract SWD. An effective homemade bait can be made by combining 1 tablespoon active dry yeast, 4 tablespoons sugar, and 1.5 cups of water. The bait should be added to the trap with a 1” depth. Alternatively, you can use a commercially available lure. The Scentry lure (Scentry Biologicals, Inc.) is a gel sachet that has catch rates comparable to the yeast-sugar bait but in a non-liquid formulation, allowing for less mess and easier identification of SWD. Non-liquid lures should be used with a 1” deep drowning solution of water and a drop of unscented soap. Check traps weekly for SWD, record the catches, and replace the liquid bait or drowning solution. Commercial lures vary in longevity, but generally will last at least four weeks.

Fig. 2. A yeast-sugar baited monitoring trap with a yellow sticky trap hung within a raspberry canopy.
Spotted wing Drosophila lures are attractive to other insects, including other vinegar flies, so it is important to know how to identify SWD. They are small (~1/10 inch in length), so identification requires a 10x hand lens. Adult flies are golden brown in color and have red eyes. Male SWD can be identified from a single dark patch near the tip of each wing (Figure 1). Wing spots darken with age, so spots may not be apparent on young male SWD. Females lack wing spots, but can be identified by their egg-laying device, which is dark in color with small tooth-like serrations (Figure 1).
Sampling for larvae in your fruit can help you monitor the fruit marketability status and to know whether management actions are working. To sample from a planting, place at least 15 ripe fruit in a plastic bag. Lightly squeeze each fruit. Add in a strong salt solution (1 cup salt to 1 gallon water). The solution should be enough to cover all of the fruit in the bag. If they are present, small white SWD larvae will emerge and rise to the top of the liquid after 30 minutes (Figure 3), and can be counted. We recommend sampling from multiple areas of your crop with emphasis on the edge and interior of your fields. Typically, the edge will have higher infestation than the interior. Having samples from multiple locations will allow you to focus management on heavily infested areas. You may not see adult SWD in traps before you find larvae in your fruit, and so we recommend monitoring for larvae and adults throughout your crop season so no unsuspected infestations develop. Visit http://goo.gl/bJf0CG for a more complete guide to the sampling process.
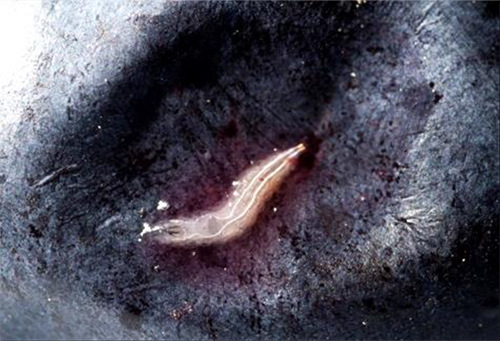
Fig. 3. A large late-instar SWD larvae on the surface of a blueberry.
Sampling Fruit for SWD Larvae
- Pick 15-25 ripe fruit.
- Lightly squeeze each fruit.
- Mix fruit with a strong salt solution (1 cup salt in 1 gallon water) in a resealable bag.
- Wait 30 minutes.
- Look for small white larvae floating on the top.
- Repeat from multiple locations in your field, especially near the field edge.
Other insects, especially native fruit flies, look similar to SWD. Thus proper identification is extremely important. You can get help with the identification of adult or larval SWD from your crop consultant or your local extension agent. The best way to monitor for SWD is to use traps for the adults and take fruit samples for larvae. However, certain types of damage on your fruit could also indicate SWD infestation. This includes soft spots or scarring on the skin, wrinkled skin, and collapsed areas on the fruit. Leaky berries is another symptom and in raspberry plantings, red patches of juice left on receptacles after picking a berry is another tell-tale sign of infestation.
If any SWD are found in traps or Drosophila larvae are found in the fruit samples, control actions should be taken immediately. Many conventional berry and cherry growers begin insecticide applications as soon as one fly is detected in their field and the fruit are ripening or ripe. Implementing your cultural controls before this detection will help keep populations from growing quickly. It is also important not to delay responding to detection of infestation as this pest can build populations very quickly.
Cultivar Selection
Planting early-ripening varieties of blueberries, including Duke, Draper, and Bluecrop can help to decrease your chances of intense SWD infestation. In raspberries, some growers have chosen to only produce a summer crop, avoiding the heavy infestation in fall raspberries altogether. June-bearing strawberries are typically at low-risk of SWD infestation, but ever-bearing strawberries have a higher risk in later parts of the summer. SWD is less successful and takes longer laying eggs in thicker-skinned fruit, so it may be beneficial to select fruit varieties with thicker skins. Grapes are not a preferred host for SWD and most grape producers have not had major issues with SWD. However, grape varieties with thinner skin or those that have splitting are more vulnerable to SWD infestation and the associated sour rot infections.
Many extension programs will provide weekly updates on SWD captures in your region. Staying connected with these updates, especially as your fruit begin to ripen, will help you better prepare. If you are in Michigan, find these reports at:www.ipm.msu.edu/invasive_species/spotted_wing_drosophila
Sanitation
Over-ripe and damaged fruit can act as a reservoir for SWD and other pests, increasing their populations and making them even harder to control. Some U-pick operations have customers collect damaged fruit as they pick, others send crews through after the customers leave to remove remaining over-ripe berries. Don’t leave waste piles of fruit in the open. They should be bagged, burned, or frozen. If bagging the fruit, use a clear trash bag and leave in the sun for at least 48 hours to kill the larvae (Fig. 4).

Fig. 4. Waste fruit disposed of in a clear trash bag.
SWD has a broad host range and will infest other non-crop plants, especially those that produce small fruits including wild raspberry, blackberry, honeysuckle, and American pokeweed. If these wild plants surround your field edge, they could act as a refuge for SWD. Early producing plants, such as honeysuckle, give SWD a place to develop and increase populations before crops ripen. Initial trials in Michigan suggest that SWD populations may increase faster in areas where honeysuckle is present. If these plants are present in high density on the edge of your crop, such as a bramble patch, removing these could decrease the onset and severity of the SWD population to your crop. However, this has not been well tested and removal of wild hosts is not guaranteed to reduce SWD populations.
Pruning and Harvest Frequency
SWD’s small size means that it is very susceptible to desiccation (drying out), so they prefer more humid areas like inside the crop canopy and low to the ground. You can use this to your advantage by keeping plants well pruned. This has the double benefit of reduced canopy humidity as well as improving spray coverage, both of which can reduce SWD survival. Harvest frequency also plays a major role in the development of SWD infestation. Harvest fruit daily or every other day to minimize infestation. Harvest intervals of three or more days significantly increase the prevalence of SWD larvae found in raspberries (Figure 5). In our trials, harvesting every 2 days provided the greatest yield per hour in raspberries.
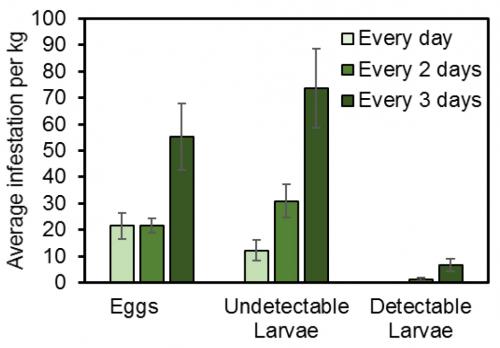
Fig. 5. The number of eggs, undetectable larvae (1st and 2nd instars) and detectable larvae (3rd instars) per kilogram of raspberries when harvested daily, every 2 days, and every 3 days.
Exclusion Netting
Physically excluding SWD flies from your crop can provide good control, and may be especially relevant for high-value crops like berries and small fruit. High tunnels or bird netting structures can both be modified for use of insect exclusion nets, but it should be at least 80 gram netting to keep flies out. Netting in our trials was purchased from Berry Protection Solutions in Stephentown, NY and manufactured by Tek-Knit Industries. We found an average reduction of 73% and a 4 week delay in SWD eggs, larvae, and adults in the netted high tunnels covered with 80 gram netting. Additionally, we’ve found that using netting in combination with insecticidal applications can provide better reduction of SWD infestation than either netting or insecticides alone. However, unless the netting is completely sealed, you can expect colonization within the protected structure and infestation of the fruit.
Access doors can be designed to best fit the needs of the farm practices (Figure 6). To allow for a tractor-mounted sprayer, barn style wooden doors can be constructed and fitted with netting. If only a backpack sprayer is required, a door sealed by a magnetic strip, zipper, or a vestibule can be constructed. Netting the perimeter of a series of high tunnels may also provide adequate control. Netting the perimeter of five tunnels (one acre) with netting is estimated to cost $6,100 (Figure 7). The lifespan of the netting is predicted to be seven years and so this cost can be amortized over these years. We also expect that with practice, labor costs would decrease.

Fig. 6. Exclusion netting fitted to a high tunnel raspberry system, with doors to allow for sprayer access.
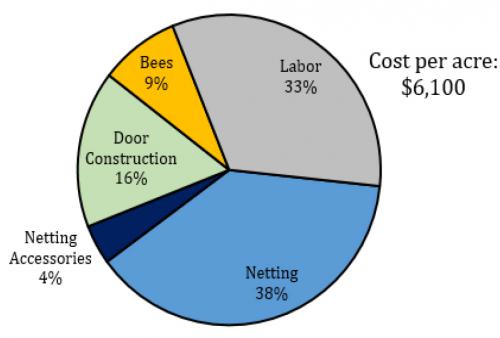
Fig. 7 The estimated cost to cover the perimeter of 1 acre of high tunnels (five 400ft tunnels) in 80 gram netting.
Holes from equipment, storms, etc. will need to be patched – taping both sides or using thick thread to sew the hole together are viable repair methods. For summer-blooming raspberries, bumble bees or other pollinators should be stocked to ensure pollination. In other crops like blueberry with spring bloom, netting can be applied after pollination is complete. In our trials in Michigan, netting had no effect on temperature or fruit quality (size, sugar content, or weight), but in warmer climates, exclusion netting has the possibility of increasing the temperature and degrading fruit quality. Exclusion netting may also provide control of other insects, birds, and some rodent pests.
Attract-and-Kill
Attract-and-kill approaches use attractants to bring the target insect to a location where they are killed. Killing can be achieved through trapping or through delivery of a contact insecticide. At present there are no labeled attract and kill devices for SWD but several are being developed by university entomologists. Research suggests that attract and kill may improve control of SWD but is likely to require a large number (500+/acre) of killing devices to impact SWD populations. This approach could be carried out by using a large number of monitoring traps and is best suited to small (<1/4 acre) plantings.
Biological Control
There are some native parasitoid wasp species and some predators that have been reported to attack SWD in the U.S., and research of this is ongoing. Currently, we have very limited knowledge of predators or parasitoid wasps that attack SWD in the Midwest. Native predation of SWD is likely to only occur at high population levels of SWD and crop infestation would still be apparent. Research is underway on the potential to introduce parasitoid wasps from Asia, SWD’s native range, for control.
Chemical Control
There are few NOP compliant insecticides that provide effective SWD control. Entrust® (spinosad) has been found to be the most effective of these, but it must also be rotated with another insecticide after two applications for resistance management. Pyganic® can be rotated with Entrust®, but has demonstrated limited efficacy and short residual for SWD. Grandevo® is another organic rotation partner that can be used with Entrust®, and this has provided variable control within the U.S. In Michigan, initial trials with Grandevo® have shown efficacious control when rotated with Entrust®.
The limited availability of effective organic SWD insecticides and label restrictions on Entrust® make spray coverage and timing of applications critical to achieving control. Sprayers should be calibrated at least once per year and appropriate spray volumes used to achieve excellent coverage. Initial research suggests that SWD are active during cooler parts of the day, in the morning and at dusk. Targeting sprays during these times may increase efficacy. When bees are present in your crop, avoid insecticide applications. If control is needed, use insecticides less toxic to bees and do not spray when they are active.
|
Entrust SC Label Restrictions by Crop | ||||
|---|---|---|---|---|
|
Crop |
Blueberry |
Raspberry, Blackberry |
Strawberry |
Stone Fruit |
|
Application Rate |
4-6 fl oz/acre |
4-6 fl oz/acre |
4-6 fl oz/acre |
4-8 fl oz/ acre |
|
PHI |
3 days |
1 days |
1 days |
7 days |
|
Retreatment Interval |
6 days |
5 days |
5 days |
7 days |
|
Max Amount |
29 fl oz per year |
29 fl oz per year |
29 fl oz per year |
29 fl oz per year |
|
Max No. Applications |
6 per year |
6 per year |
5 per year |
3 per year |
Post-Harvest Cooling
If fruit are suspected of having some infestation by SWD after being harvested, they should be cooled as soon as possible to stop further development of the insect. Cooling to 35°F will stop the development of the eggs and larvae inside the fruit, which can prevent them from becoming larger and noticeable. Cooling the fruit for three days has been shown to kill SWD larvae. If your fruit is sold directly to consumers, you should advise them to keep it in the refrigerator. Freezing the fruit will kill eggs and larvae of SWD.
Summary
We emphasize that controlling SWD requires a rigorous, persistent, and diverse management plan. Using as many possible control techniques on your farm will help to reduce SWD infestation. For effective management, follow these key points:
- Monitor fields with traps and check them weekly, especially when fruit is ripening.
- Monitor SWD infestation by testing ripe fruit samples in a salt solution before and during harvest.
- Take management actions when SWD are detected and fruit are ripening or ripe. This includes increasing your harvest frequency, keeping your fields well-pruned and clean, removing and destroying leftover fruit, and using recommended NOP compliant insecticides.
- Track the effectiveness of your management programs with continual monitoring throughout the season.
- Stay informed with your regional SWD counts and new management techniques using the resources below.
References and Resources
For more information, check the Michigan State University SWD page online at www.ipm.msu.edu/SWD.htm
For identification help, contact your local extension agent or find keys at www.ipm.msu.edu/SWD.htm
For research updates, go to www.spottedwing.org
This fact sheet was produced with support from USDA-SCRI TunnelBerries project supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under Agreement No. 2014-51181-22380, the USDA-NIFA Organic Agriculture Research and Extension Initiative Grant Agreement No. 2015-51300-24154, and the North Central Region Sustainable Agriculture Research and Education program Award 2014-38640-22156.
MSU is an affirmative-action, equal-opportunity employer, committed to achieving excellence through a diverse workforce and inclusive culture that encourages all people to reach their full potential. Michigan State University Extension programs and materials are open to all without regard to race, color, national origin, gender, gender identity, religion, age, height, weight, disability, political beliefs, sexual orientation, marital status, family status or veteran status. Issued in furtherance of MSU Extension work, acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture. Ray Hammerschmidt, Interim Director, MSU Extension, East Lansing, MI 48824. This information is for educational purposes only. Reference to commercial products or trade names does not imply endorsement by MSU Extension or bias against those not mentioned.



 Print
Print Email
Email