Making the most out of your soil test
What does soil pH mean for your garden?

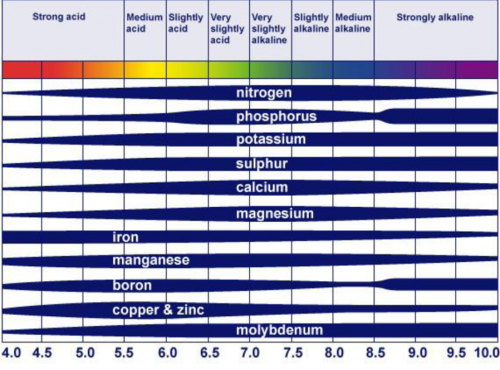
A garden soil’s pH is a measure of its acidity or alkalinity. This is important to know when growing plants because pH influences the chemical and biological reactions that occur in the soil, including the availability and uptake of essential plant nutrients. Plants have different nutrient needs. Some species such as rhododendron or blueberry perform best with a more acidic pH level; others, such as lawn grasses, prefer a more neutral or alkaline pH.
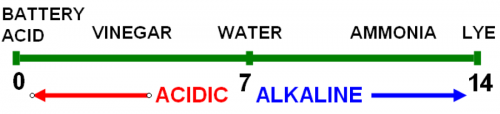
Soil pH is measured on a 14-point scale that runs from most acidic (1) to most alkaline or basic (14). A pH of 7 describes a “neutral” pH, meaning that alkalinity and acidity are equally balanced. Michigan soils can range from 4.5 to 8.5, depending on the soil’s parent material and the environmental or cultural influences of previous crops grown. For instance, if a homeowner applies lime to a lawn every year, the soil pH is likely to be above 7, even if the parent soil is naturally more acidic (less than 7 on the pH scale).
Changing pH
It is important to remember that not all soils are created equal. Depending on a soil’s parent material, organic matter, temperature and moisture, changing soil pH may take time. Soils have the ability to buffer or resist changes. Also, changing pH by one point is not as difficult as changing it two points.
Products containing limestone or sulfur can help raise or lower soil pH, respectively. Be aware that too much of any product can work against an effort to improve a plant’s growing environment. To quickly alter the soil pH, work the amendment into the soil to the depth of around 6 inches. Where this is not possible (as in established lawns, orchards, or landscapes), soil surface applications are necessary. In this case, the resulting change will take one or more seasons.
While soil pH increase can occur rather quickly using certain lime products, it may take several months for sulfur to provide the same acidifying result. Soil microorganisms play an important role in converting sulfur to acid and this reaction also depends on temperature and moisture.
 Raising soil pH
Raising soil pH
When soil pH is too low (acidic), lime can be used to neutralize or raise the pH level. Products like dolomitic or calcitic limestone, hydrated lime and even wood ash have different chemical properties and can be used to accomplish different things in the garden soil. Limestone products are also useful as a source of calcium when this nutrient is too low (which can occur in sandy soils). Apply products as recommended by your soil test report and incorporate them to a depth of 6 inches when possible.
Lowering soil pH
If the soil pH is too high (alkaline), it can be adjusted by applying sulfur to the soil. Products commonly available include elemental or ground sulfur, iron sulfate and aluminum sulfate. When needed, most plants will benefit from using any of these products. However, a few plants are a little more specific in their requirements. To lower the pH for rhododendron, use ground or elemental sulfur or iron sulfate since aluminum can be toxic to rhododendron. When blue hydrangea flower color is desired, lowering the pH with aluminum sulfate is best since the aluminum helps to enhance blue color. Apply products as recommended by your soil test report. If a sulfur product comes into contact with plant leaves, it should be promptly rinsed off.
Other things that affect nutrient availability
Some essential plant nutrients (such as nitrogen) are less available in cold or water-logged soils. Plants exhibiting nutrient deficient symptoms may actually be unable to get the nutrients that are already in the soil. Lawns that are slow to green up during a prolonged cold spring do not need additional of fertilizer applied to correct a sometimes “off-green” appearance. Only time and temperature can correct this situation.
Download a printable tip sheet of this aricle: Making the Most out of Your Soil Test



 Print
Print Email
Email


